
Empirical Exercise 4 in R
In this exercise, we’re going to replicate the difference-in-differences analysis from
Does a ban on informal health providers save lives? Evidence from Malawi
by Professor Susan Godlonton and Dr. Edward Okeke. The authors estimate the impact of Malawi’s 2007 ban
on traditional birth attendants (TBAs) on a range of birth outcomes. At the end of the exercise, we’ll export our
regression results to excel using openxlsx, as we did in Empirical Exercise 3.
Getting Started
The data set E4-GodlontonOkeke-data.dta contains information (from the
2010 Malawi Demographic and Health Survey)
on 19,680 live births between July 2005 and September 2010. Each observation represents a birth. Create
R script that opens the Stata data set in R (using read_dta() from haven). You should have received the data set over email, and
you will need to save it and load it to R from your computer. Your code for starting the script should look something like:
## ECON 325: In-Class Activity 4
## A. Student
# libraries
library(tidyverse)
library(fixest)
library(openxlsx)
library(haven)
# file path
mypath <- "C:/Users/ECON523/data"
## load data
e4data <- read_dta(paste0(mypath, "/E4-GodlontonOkeke-data.dta"))
Make sure to install the packages tidyverse, fixest, openxlsx, and haven if you have not already done so.
In-Class Activity
Question 1
To implement difference-in-differences, we need to add the following columns/variables to the e4data data frame:
- a dummy variable for the post-treatment period,
- a dummy variable for the treatment group, and
- an interaction between the two
The post variable is already present in the data set, one of the columns of e4data. What is the mean of post? What fraction of the observations in the data set occur in the post-treatment period?
Question 2
The time variable indexes the month and year in which a birth took place. Unfortunately, it is in Stata’s date format, and reads into R without the appropriate labels. Fortunately, the same information is contained in the variables birthyr and birthmonth. Cross-tabulate birthyr and post to see how Professor Godlonton and Dr. Okeke define the post-treatment time period in their analysis. What is the first treated month?
Hint: you can use xtabs(~ y + x, data = df) to cross-tabulate the columns y and x in data frame df.
Question 3
We need to define an indicator for the treatment group. Professor Godlonton and Dr. Okeke define the treatment group as DHS clusters (i.e. communities) that were at or above the 75th percentile in terms of use of TBAs prior to the ban. Data on use of TBAs comes from responses to the question below:

Responses have been converted into a set of different variables representing the different
types of attendants who might have been present at the birth. Tabulate (using count(df, varname))
the m3g variable, which indicates whether a woman indicated that a TBA was present at a birth. What pattern of responses do you observe?
Hint: a value of 0 indicates no, 1 indicates yes, and 9 indicates don’t know or a refusal to answer.
Question 4
We want to generate a dummy variable that is equal to one if a TBA was present at a particular birth, equal to zero if a TBA was not present, and equal to missing if a woman did not answer the question about TBAs.
There are several different ways to do this. The code below illustrates a simple approach using if_else.
e4data$tba <- if_else(e4data$m3g == 9, NA, e4data$m3g)
This generates a new variable, tba, that is the same as the m3g variable except that tba is equal to missing for all
observations where m3g is equal to 9. (It is usually better to generate a new variable/column
instead of modifying the raw data, because you don’t want to make mistakes that you cannot undo.)
Question 5
We want to generate a treatment group dummy - an indicator for DHS clusters where use of TBAs was at or above the 75th percentile prior to the ban. How should we do it?
The variable dhsclust is an ID number for each DHS cluster. How many clusters are there in the data set?
We can use group_by(dhsclust) before generating the conditional mean in the pre-treatment period, as the code below illustrates:
e4data <- e4data %>%
group_by(dhsclust) %>%
mutate(meantba = mean(tba[post == 0], na.rm = TRUE)) %>%
ungroup()
Question 6
The code below illustrates how to define a scalar cutoff equal to the 75th percentile
of the variable meantba.
cutoff <- quantile(e4data$meantba, probs = 0.75, na.rm = "TRUE")
Add this to your code and then create a new column/variable (in the e4data data frame) called high_exp that is an indicator
for DHS clusters where the level of TBA use prior to the ban exceeded the cutoff we just calculated. Replace this variable with NA
for observations where data on the use of TBAs is missing (i.e. when m3g equals 9). What is the mean of high_exp?
Question 7
The last variable we need to conduct difference-in-differences analysis is an interaction between
our treatment variable, high_exp, and the post variable. Generate such a variable as a column
in the e4data data frame. I suggest calling it highxpost.
Question 8
Now you are ready to run a regression. Regress the tba dummy on high_exp, post, and
highxpost. What is the difference-in-differences estimate of the treatment effect
of the TBA ban on use of informal birth attendants? How do your results compare
to those in Table 5, Panel A, Column 1 of the paper?
Question 9
You are using the same data as Professor Godlonton and Dr. Okeke, so you should be able to replicate their coefficient estimates and standard errors exactly. Have you done it?
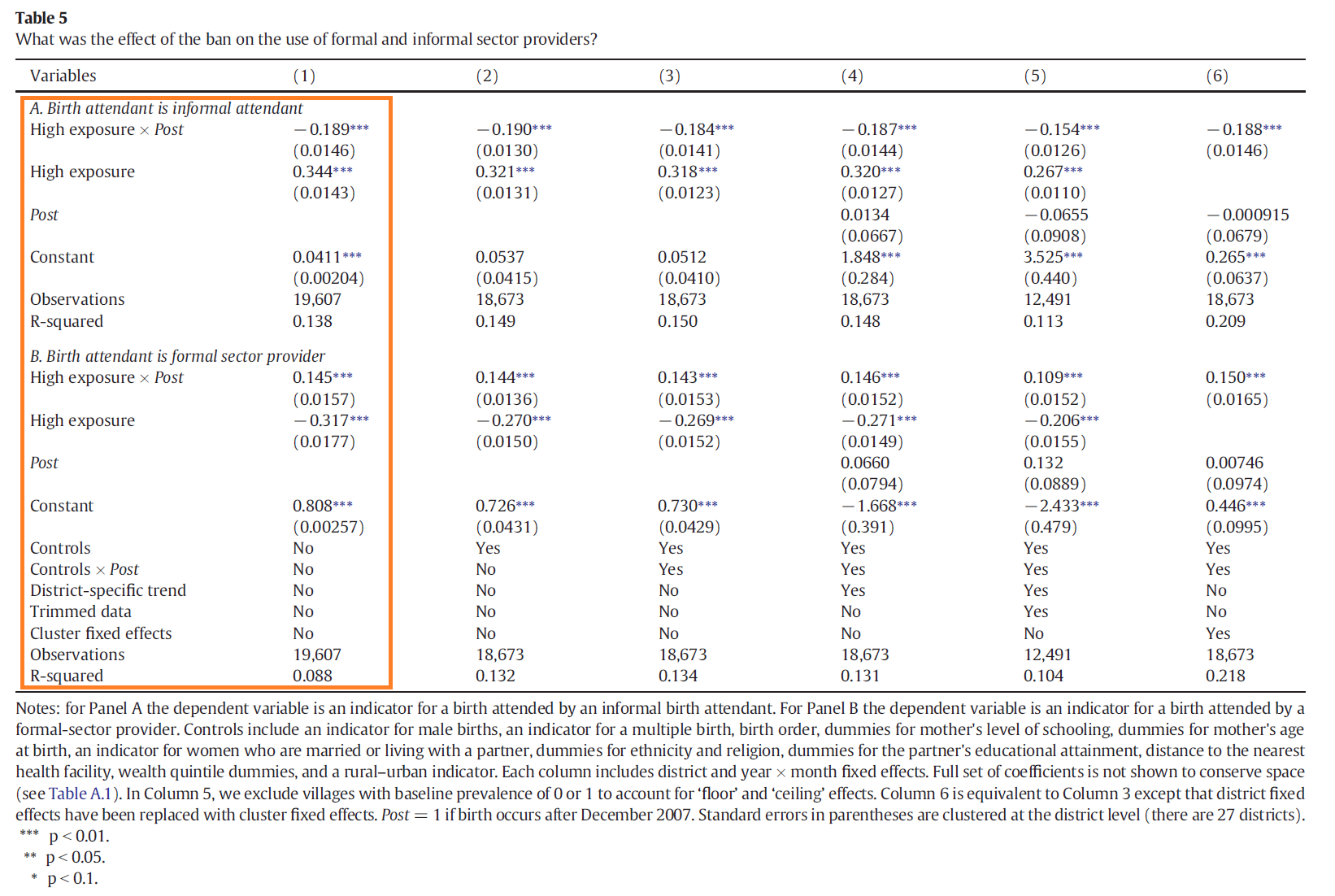
Read the notes below Table 5. See if you can modify your regression command so that your results are precisely identical to those in the paper.
Empirical Exercise
Start by generating a new R script that loads E4-GodlontonOkeke-data.dta and uses your answers
to the in-class activity to generate the variables needed to replicate Column 1 of
Table 5. Then add the following code, which defines a program reshape_regs() that takes output from
a regression (estimating using feols()), cleans it up using the tidy() function form the broom package,
and then reformats the results to look like a column of a regression table.
reshape_regs <- function(myresults){
olsresults <- tidy(myresults)
olsresults$index <- 1:nrow(olsresults)
olsresults <- olsresults %>%
mutate(stars = if_else(p.value <= 0.01, "***",
if_else(p.value <= 0.05, "**",
if_else(p.value <= 0.1, "*", "")))) %>%
mutate(across(c(estimate, std.error), ~ sprintf("%.3f", .))) %>%
mutate(across(c(estimate, std.error), ~ as.character(.))) %>%
mutate(estimate = str_c(estimate, stars)) %>%
mutate(std.error = str_c("(", std.error, ")")) %>%
select(term, estimate, std.error, index) %>%
pivot_longer(c(estimate, std.error), names_to = "type", values_to = "est") %>%
select(term, type, est)
return(olsresults)
}
If you have not already, install the package broom and then add broom to the list of libraries
that you load at the start of your script.
Question 1: Replicating Column 1 from Tables 5 and 6
Part (a)
Use feols() to estimate a difference-in-differences specification that replicates Table 5, Panel A,
Column 1. Then use the program we defined above to clean up your results and store them in the data frame c1,
as illustrtated in the code below:
T5AC1 <- feols(tba ~ highxpost + high_exp | district + time, data = e4data, vcov = ~district)
c1 <- reshape_regs(T5AC1)
print(c1)
You can also use the code below to store the R-squared and the number of observations as character strings that can be added to the results table later.
N1 <- as.character(T5AC1$nobs)
R2_1 <- as.character(round(r2(T5AC1)[2],3))
Part (b)
Now replicate Table 5, Panel B, Column 1 (the same specification with the sba dummy
as the outcome variable) and store your results.
Part (c)
Recode the m3h variable to generate a dummy for having a friend or relative
as the birth attendant. Use this variable to replicate Table 6, Panel A,
Column 1. Store your results.
Part (d)
Now generate a variable alone that is equal to one minus the maximum of
the tba, sba, and friend variables. Use this variable to replicate
Table 6, Panel B, Column 1. Store your results.
Hint: use pmax() to calculate the within-row maximum across multiple columns.
Part (e)
Use left_join() to combine the results from your four regression specifications. The code below
illustrates the first step, combining the results from the first two regressions into a single data frame.
results1 <- left_join(c1, c2, by = c("term", "type"))
print(results1)
Once you have combined all four regression specifications into a single data frame,
drop the type column (which was helpful when merging the results from the different regressions),
modify the variable labels in the term column, and rename the term column so that it has no column name. You should also
use add_row() to add rows containing the number of observations in each specification and the R-squared. If you want,
you can also add rows indicating which fixed effects are included.
Once you have a nicely formatted data frame, export it to excel by modifying the code from Empirical Exercise 3.
Question 2: Assessing the Common Trends Assumption
Next, assess the validity of the common trends assumption by replicating the first two columns of Table 2 (we don’t have the outcome data needed to replicate Columns 3 and 4).
Part (a)
Drop the observations from after the ban was in place. Then, interact the time variable, which indexes the month of birth, with the high_exp variable. This should
give you all the variables needed to replicate the first two columns in Table 2.
Part (b)
Replicate columns 1 and 2 from Table 2 to the best of your ability. Store your results.
Part (c)
Export your results to excel as a nicely formatted table (all of the guidance from Question 1 still applies).
Additional Activities
If you are looking for ways to expand your program evaluation skills further, extend your answer to Question 1 by including district-specific time trends,
as Professor Godlonton and Dr. Okeke do in Columns 4 through 6 of Tables 5 and 6. Alternatively, you can replicate the main analysis
using a continuous measure of treatment intensity: the interaction between the level of TBA use prior to the ban and the post dummy. Generate
this new treatment variable using your existing meantba variable, and then estimate regressions that control for meantba and its interaction
with post. How do the results from these alternative specifications compare to those reported in the paper? Finally, consider using DHS cluster fixed effects
and month of birth fixed effects in the same specification. Do the DHS cluster fixed effects reduce the standard errors?
This exercise is part of the module Diff-in-Diff in Panel Data.